Abstract
Introduction: Since the implementation of next generation sequencing techniques, inherited mutations in malignancy-associated susceptibility genes have become of major interest, to identify high-risk individuals, even before an actual disease onset. One challenge in this regard is that germline susceptibility variants often display incomplete or reduced penetrance, which can be influenced by genetic as well as environmental factors (Martin-Lorenzo A. et al., Cancer Discov,, 2015), making it even harder to assess the complete spectrum of the predisposition. Here, we aimed to elucidate the variable penetrance of a family, which harbors the inherited B-cell precursor ALL (BCP-ALL) susceptibility variant PAX5 c.547G>A (Auer F et al., Leukemia, 2014).
Methods: We generated a new in vivo model, namely double transgenic Bank1+/-+Pax5+/- mice. Next generation sequencing was used to determine candidate genes influencing the variable penetrance of PAX5 c.547G>A. Pax5+/-+Bank1+/-, as well as Arf-/-+Bank1+/- mouse models were utilized to assess the tumor suppressor potential of Bank1. Murine leukemias were characterized by immune-phenotyping, whole exome sequencing (WES) as well as expression analyses.
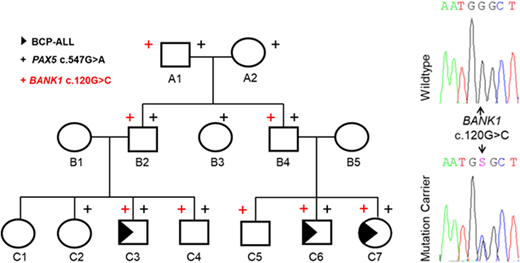
Results: In a family, harboring PAX5 c.547G>A, 5 out of 7 analyzed children were shown to be mutation carriers, while only 3 out of 5 developed BCP-ALL. In order to assess this variable penetrance further, re-analysis of the WES data revealed an additional rare variant in the B-cell scaffold protein with ankyrin repeats (BANK1) (c.120G>C; rs35978636, minor allele frequency (MAF)<0.01), with a similar transmission spectrum like the heterozygous PAX5 susceptibility variant c.547G>A (Figure 1).
BANK1 is primarily expressed in B-cells where it acts as an important adaptor that is involved in B-cell receptor (BCR) induced Ca2+ mobilization from intracellular stores (Yokoyama K et al., EMBO, 2002). Importantly, single nucleotide variants (SNVs) in BANK1 could be linked to confer a susceptibility to a variety of autoimmune diseases, including Systemic Lupus Erythematosus (SLE) (Kozyrev SV et al., Nat. Genet., 2008). The identified BANK1 variant c.120G>C is located in Exon2, the IP3R binding site of BANK1, in proximity to the previously described SLE susceptibility variant c.182G>A, and results in a predicted deleterious protein structure as calculated by SIFT and PolyPhen-2.
To test whether Bank1 and Pax5 heterozygosity could cooperate to promote BCP-ALL development, we crossed Bank1+/- mice on a Pax5 heterozygous background and monitored the leukemia incidence of double transgenic Bank1+/-+Pax5+/- mice. Bank1+/-+Pax5+/- mice developed BCP-ALL with clonal blast infiltration in secondary lymphoid organs and an identical leukemia phenotype (CD19-B220+IgM-) and latency like Pax5+/- mice. However, we observed an increase in BCP-ALL disease incidence of 33% compared to the Pax5+/- cohort. These results suggest that Pax5 loss promotes an aberrant B-cell precursor compartment that is susceptible for leukemic transformation, while the combination with Bank1 heterozygosity causes a susceptibility increase in the pre-leukemic population. While these results reflect the variable BCP-ALL penetrance in the human family carrying both PAX5 c.547G>A and BANK1 c.120G>C, it also underlines the multifactorial nature of the disease.
Since these results suggest a tumor suppressor function of BANK1 in BCP-ALL, we next explored the effect of Bank1 loss of function on the disease phenotype in a tumor-prone mouse model lacking BCP-ALL susceptibility. Therefore, Bank1-/- mice were crossed back on a p19Arf-deficient background, to obtain the mouse cohorts Bank1+/-p19Arf-/- and Bank1-/-p19Arf-/-. The additional Bank1-deficiency promoted a shift from a T- to mainly B-cell phenotype in the resulting p19Arf-deficient tumors, further supporting a specific tumor suppressor role of Bank1 in BCP-ALL.
Conclusion: Taken together, our data underline a tumor suppressor role of Bank1 in the context of BCP-ALL development and supports its involvement in the variable penetrance of BCP-ALL in a family carrying PAX5 c.547G>A.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal